| HERE IS A GALLERY OF IMAGES WHICH I OBTAINED EXPERIMENTALLY IN DIVERSE EXPERIMENTS |
|
A pentagonal copper crystallite. This little "crystal" is actually a macle. It is the association
of five single crystals, which join along lines of crystallographic mismatch (so-called re-entrance angles).
The origin of these macles is in the shape of the initial crystal germ.
It is likely that a regular spacefilling, like six-fold structure should be the stable crystalline lattice.
But this view is only true thermodynamically, for a complete lattice, in an abstract space where the entire lattice
would be formed "all of a sudden" out of nowhere, and globally.
In the real world, crystals grow, and they start off by a single nucleus, may be a single atom, then two, then 3,
then 4 then 5 etc.
For such small clusters, the six-fold symmetry, the space-filling pattern, may not be the stable configuration, it may not even be defined.
Now, it turns out that growth of facetted crystals requires nucleation events on each facet in order to grow one new facet.
This is to say that, like onion skins, each new layer is difficult to grow, because you need a new nucleation event on each
facet.
...Actually, this is true only of regular space-filling crystals. A kinetic study shows that a pentagonal nucleus requires only
a single nucleation
event to grow a new layer. This is a subtle geometrical fact, related to how the atoms are positioned along corners of the crystals.
In a hexagonal or a cubic lattice, a new nucleation event is required along each facet,
because the wave of depositing crystals on each facet cannot propagate from one facet to an other, when it has reached the edge
(it cannot turn the corner).
While on pentagonal germs, the crystallization wave along one facet can propagate "around the corners". Therefore,
pentagonal macles are kinetically favored.
For me, animals are some sorts of soft macles. This is to say that exactly the same physical rules, may select a
complex 3D form, composed, in detail, of the association of parts in which all the positions of the entities are not equivalent,
although they follow a single
physical rule. An example of consequence, is that the tetrapod body may be viewed as a 4-fold macle, whose pattern
is inherited from the germ of 4 cells which propagates "upwards"
in the form of the tetrapod body.
An other fascinating macle is the banana, which has 3 fold symmetry, and can be easily split along the planes of
3 fold-symmetry (you should try next time you eat a banana).
|
 |
| More elongated copper crystals obtained during electrochemical
growth. These growths last about 2 days, for about 200 micrometers of growth.
|
 |
|
Now this is what gives a very rapid electrochemical growth, which lasts only a few seconds.
Please note the scale (one twentieth of a millimeter) |
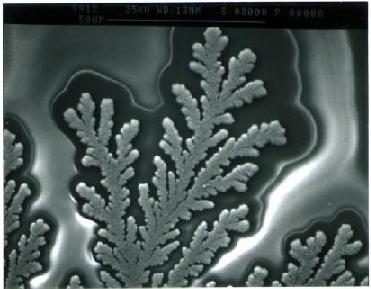 |
|
Branching electrochemical deposit, obtained in a few minutes.
(These experiments are performed in thin layer electrochemical cells).
The image shows a complete pattern, approx. 3 centimeters wide |
 |
|
Transition of morphology, in radial cells, between branching patterns and radial filaments. |
 |
| Planar branching morphology on glass slides. (Width about 3 mm).
|
 |
|
Branching growth observed in presence of random scratches
(Width approx. 3mm.) . |
 |
| Planar branching deposits, in presence of scratches.
(the parallel lines are not actually ordinary scratches, they were done on purpose along the glass
by sliding a teflon piece at high temp.)
(Width 3mm approx.) |
 |
|
High temperature (circa 500°C) of an Al mirror on quartz.
I had the pleasure to work on this topic with Lazlo Balasz. Si clusters crystallize from diffusing Si.
The diffusing atoms are produced by a reduction of SiO2 by Al, forming Al203.
|
 |
| Oxidation of an aluminum mirror,
same as before, except that the growth starts from an edge (so-called channel geometry, in DLA growth).
Width 3 millimeters. |
 |
| Oxidation of an aluminum thin film, on glass,
around a point like defect.
(Width approx. 0,5 mmm) |
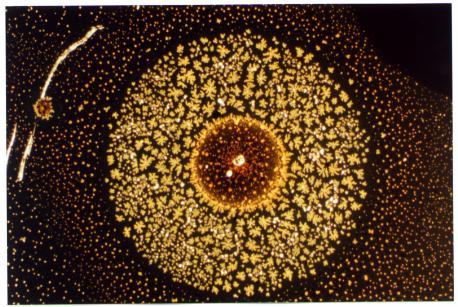 |
| Oxidation of a carbon film on glass. (Width 0,5 mm) |
 |
| Oxydation of an aluminum film on glass, at a higher temperature
(>600 degrees) At this temperature, the film is partially melt.
(see the drops).(Width 0,5 mmm.) |
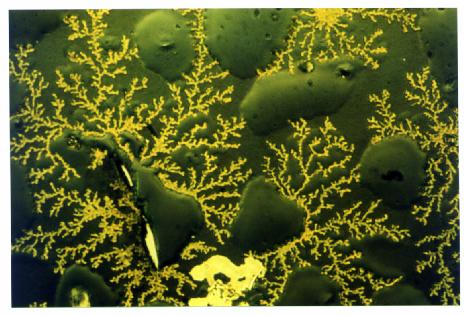 |
Go to historical images and miscellani
Voir les images
|
Retour à la page de présentation-Back to front page |












